Buphenyl, an FDA-approved medication for hyperammonemia, may protect memory and prevent the progression of Alzheimer’s disease. Hyperammonemia is a life-threatening condition that can affect patients at any age. It is caused by abnormal, high levels of ammonia in the blood.
Studies in mice with Alzheimer’s disease (AD) have shown that sodium phenylbutyrate, known as Buphenyl, successfully increases factors for neuronal growth and protects learning and memory, according to neurological researchers at the Rush University Medical Center.
Results from the National Institutes of Health funded study, recently were published in theJournal of Biological Chemistry.
“Understanding how the disease works is important to developing effective drugs that protect the brain and stop the progression of Alzheimer’s disease,” said Kalipada Pahan, PhD, the Floyd A. Davis professor of neurology at Rush and lead investigator of this study.
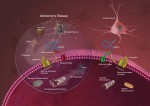
Researchers discovered the drug Buphenyl increased neurotrophic factors from astroglia. It also stimulated CREB, a memory related protein using PKC and increased neurotrophic factors throughout the brain. The diagram compares neuronal damage in Alzheimer’s disease to healthy neurons. Credited to National Institute on Aging/NIH.
A family of proteins known as neurotrophic factors help in survival and function of neurons. Past research indicates that these proteins are drastically decreased in the brain of patients with Alzheimer’s disease (AD).
“Neurotrophic factor proteins could be increased in the brain by direct injection or gene delivery,” said Pahan. “However, using an oral medication to increase the level of these protein may be the best clinical option and a cost effective way to increase the level of these proteins directly in the brain.”
“Our study found that after oral feeding, Buphenyl enters into the brain, increases these beneficial proteins in the brain, protects neurons, and improves memory and learning in mice with AD-like pathology,” said Pahan.
In the brain of a patient with AD, two abnormal structures called plaques and tangles are prime suspects in damaging and killing nerve cells. While neurons die, other brain cells like astroglia do not die.
The study findings indicate that Buphenyl increases neurotrophic factors from astroglia. Buphenyl stimulates memory-related protein CREB (cyclic AMP response element-binding protein) using another protein known as Protein Kinase C (PKC) and increases neurotrophic factors in the brain.
“Now we need to translate this finding to the clinic and test Buphenyl in Alzheimer’s disease patients,” said Pahan. “If these results are replicated in Alzheimer’s disease patients, it would open up a promising avenue of treatment of this devastating neurodegenerative disease.”
Notes about this Alzheimer’s disease research
Other researchers involved in this study are Grant Corbett, neuroscience graduate student at Rush and Avik Roy, research assistant professor at Rush.
Alzheimer’s disease is an irreversible, progressive brain disease that slowly destroys memory and thinking skills, and eventually even the ability to carry out the simplest tasks. In most people with Alzheimer’s, symptoms first appear after age 60. Alzheimer’s disease is the most common cause of dementia among older people. Alzheimer’s disease affects as many as 5.3 million Americans.
This study has been supported by grants from National Institutes of Health (AT6681, NS64564 and NS71479). GTC is supported by a grant from the NIA (T32 AG00269).
Contact: Deb Song – Rush University Medical Center
Source: Rush University Medical Center press release
Image Source: The progression of Alzheimer’s disease image is credited to the National Institute on Aging/NIH and is available in the public domain.
Original Research: Full open access research (PDF and HTML) for “Sodium phenylbutyrate enhances astrocytic neurotrophin synthesis via PKC-mediated activation of CREB: Implications for neurodegenerative disorders” by Grant Corbett, Avik Roy and Kalipada Pahan in Journal of Biological Chemistry. Published online February 12 2013 doi: 10.1074/jbc.M112.426536